Background:
Patients with acute myeloid leukemia (AML) frequently have poor-quality end of life (EOL) care including intensive care unit admissions and prolonged hospitalizations. Prognostic uncertainty and restrictions on transfusions during hospice are barriers that prevent patients with AML from having access to palliative care services. There is a paucity of data on EOL care on patients with AML that are unable to continue a venetoclax and hypomethylating agent (HMA-ven) treatment.
Methods:
A query was conducted using Northwell Cancer Institute's electronic health records to identify all transplant-ineligible patients with AML who received HMA-ven between January 2019 and December 2022 that had died. We retrospectively analyzed the charts of 84 patients who fit the criteria. EOL outcomes including rate of advanced care planning, location of death, and resource utilization were recorded. Overall survival (OS) was defined as the time from start of the last line of chemotherapy to death for patients that had disease-modifying treatment at EOL. For those that continued to best supportive care (BSC), OS was defined as the time from stopping the last dose of chemotherapy to death. Hospice length of stay (HLOS) was the time from a hospice referral to death for patients that chose to stop transfusions and pursue hospice. Kaplan-Meier method and log rank testing were used to compare time to event data.
Results:
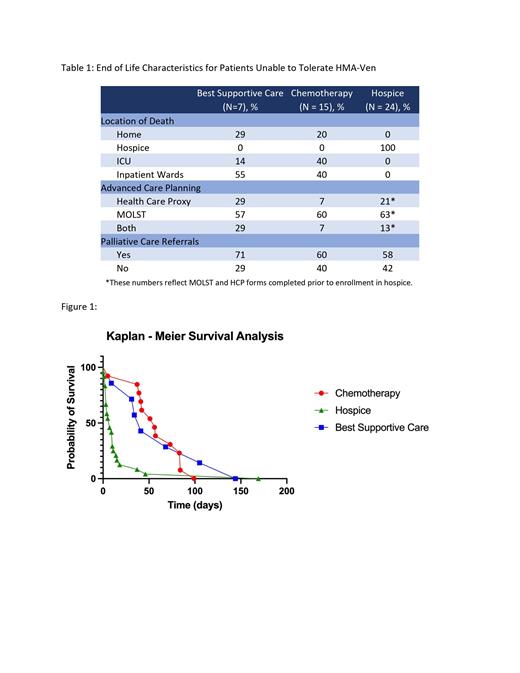
In our cohort of 84 patients, 43% of patients died on hospice, 53% of patients died in the hospital, with 23% requiring an ICU admission at the end of life. 18% had either cardiopulmonary resuscitation (CPR) or an activation of the emergency response system within 24 hours of death. While more than half of patients opted to not have CPR, 15% of these decisions were made within the last 24 hours of life. Furthermore, of the patients only 65% saw a palliative care physician while admitted for treatment. Of note, 42 patients tolerated HMA-ven and were continued to take this medication until their death.
However, 42 patients stopped HMA-Ven due to progression of disease (69%) or intolerance (31%). Of this cohort, 15 patients continued to subsequent line therapy following HMA-ven with a median OS of 56 days. 24 patients continued on to hospice without transfusion support, with a median HLOS of 7 days. Seven patients continued BSC with median OS of 41 days, requiring an average of 1.6 transfusions weekly. Of note, the patients that continued BSC did not transition to hospice as these patients were unable or unwilling to stop transfusions. There was no statistical difference in OS between receiving subsequent chemotherapy and BSC at EOL (p = 0.40). Patients receiving BSC had a lower percentage of patients dying in the ICU (55% vs 40%) and documented advanced directives with a health care proxy (29% vs 7%) compared to those that had received chemotherapy at EOL.
Discussion:
Our study identified opportunities to improve EOL care and goal-concordant care in patients with AML receiving non-curative therapy. A subset of this population significantly benefitted from transfusion-only support and stopping disease modifying treatment. Opting for BSC may drastically improve the EOL quality by decreasing ICU deaths and improving access to goal-concordant care. Further research on the timing of best supportive care to patients with AML undergoing non-curative intent therapy as opposed to continuing subsequent line chemotherapy regimens is needed.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal